how to calculate protons neutrons and electrons worksheet
how to calculate protons neutrons and electrons worksheet
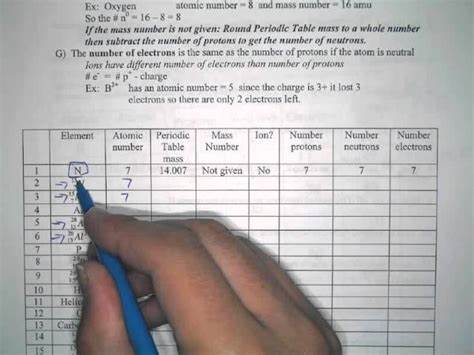
To calculate the number of protons, neutrons, and electrons for an element, you need to understand the structure of an atom. Here are the steps:
-
Determine the Atomic Number:
- The atomic number (�) is the number of protons in an atom.
- You can find the atomic number on the periodic table. It's usually located above or below the element's symbol.
-
Determine the Mass Number:
- The mass number (�) is the sum of protons and neutrons in an atom.
- You can find the mass number on the periodic table. It's usually located below the element's symbol.
-
Calculate the Number of Protons (�):
- The number of protons is equal to the atomic number.
- �=�
-
Calculate the Number of Neutrons (�):
- The number of neutrons is calculated by subtracting the number of protons from the mass number.
- �=�−�
-
Calculate the Number of Electrons (�):
- For neutral atoms, the number of electrons is equal to the number of protons.
- �=�
Here's a simple worksheet example:
Element: Carbon (C)
Atomic Number (Z): 6
Mass Number (A): 12
1. Number of Protons (P) = Z = 6
2. Number of Neutrons (N) = A - Z = 12 - 6 = 6
3. Number of Electrons (E) = Z = 6
Remember, these calculations are for neutral atoms. If the atom is an ion (charged), the number of electrons may differ from the number of protons based on the ion's charge.
-
How do you calculate protons, neutrons, and electrons?
- Protons: Equal to the atomic number (Z).
- Neutrons: Subtract the atomic number (Z) from the mass number (A).
- Electrons: Equal to the atomic number (Z) for neutral atoms.
-
How do you find the number of protons, neutrons, and electrons present in [an atom]?
- Identify the element and find its atomic number (Z) on the periodic table.
- Determine the mass number (A) of the isotope.
- Use the formulas:
- Protons (�): �
- Neutrons (�): �−�
- Electrons (�): � for neutral atoms.
-
How do you find the number of protons?
- The number of protons is equal to the atomic number (�) of the element.
-
How do you find neutrons?
- Neutrons are calculated by subtracting the atomic number (�) from the mass number (�): �=�−�.
-
How do you calculate electrons?
- For neutral atoms, the number of electrons is equal to the atomic number (�).
-
How do you calculate atoms?
- Atoms are counted in terms of moles. Avogadro's number (6.022×1023 atoms/mol) is used to convert between moles and atoms.
- The number of atoms (�) is calculated using the formula: �=Mass (g)Molar mass (g/mol)×6.022×1023.
Remember, these calculations are applicable to neutral atoms. If the atom is an ion, the number of electrons may differ based on the ion's charge.